 Thank you for visiting the Ko Laboratory page, a place for junior scientists who have a passion for science. This is their laboratory to achieve their goals for learning scientific skills, experimenting with new ideas, growing to excellency, and developing new knowledge for all. These goals are achieved by working on a few projects.
Thank you for visiting the Ko Laboratory page, a place for junior scientists who have a passion for science. This is their laboratory to achieve their goals for learning scientific skills, experimenting with new ideas, growing to excellency, and developing new knowledge for all. These goals are achieved by working on a few projects.
One of the main themes of the laboratory is to elucidate the mechanism governing the breakdown and rupture of the follicle during the ovulatory process. This process is activated by a surge of luteinizing hormone (LH), a pituitary gonadotropin, which triggers dramatic changes in the ovary at molecular, biochemical, and physical levels, eventually leading to the rupture of a mature follicle, releasing an egg. However, many of the key factors involved in and the mechanism governing the process of LH release and follicle rupture are yet to be unveiled.
Our research team aims to reveal the LH-induced intraovarian molecular events and the roles that ovarian steroid hormones play in this process, ultimately to help women who suffer from infertility caused by ovulatory defects. Other exciting projects include reproductive toxicology, novel roles that sex steroids play in reproductive/non-reproductive tissues and generation of novel transgenic animals and databases for shared uses.
Please take a moment to explore our projects in the tabs below or see some funny or serious photos taken by our team members in the gallery below.
Our Principal Investigator is CheMyong Ko.
Please contact any of our team members if you are interested in our projects or want to collaborate with us. You can find their email contacts and projects at our website here.









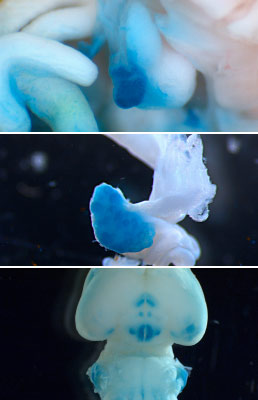 Our lab generates genetically modified (or transgenic) animal models. Genomic DNA sequences are modified using a variety of genetic manipulation procedures in vitro. The DNA sequences are then introduced into the genomes of recipient animals, creating new animals that never existed before. New animal models are continuously generated and used in our laboratory.
Our lab generates genetically modified (or transgenic) animal models. Genomic DNA sequences are modified using a variety of genetic manipulation procedures in vitro. The DNA sequences are then introduced into the genomes of recipient animals, creating new animals that never existed before. New animal models are continuously generated and used in our laboratory.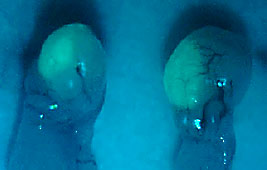 Female reproductive senescence begins before birth and continues throughout a lifetime, culminating with menopause, the state of depletion of ovarian follicles. The female reproductive axis is unique in that it reaches a senescent state when other organs in the body are generally healthy. On average, women reach menopause at 50 years of age whereas their life expectancy is over 80 years. Hence, the majority of women will spend, or are spending, more than one-third of their life in a post-menopausal state. Epidemiological studies show that late onset of menopause confers longevity and decelerates the appearance of much age-related morbidity, suggesting that delaying the onset of menopause will improve the health of aging women and therefore their quality of life. In this study, using animal models, we aim to develop an experimental procedure that will be conceptually applicable to delaying the natural onset of menopause in women. Once developed and successfully executed, this novel procedure may delay the onset of menopause by multiple years so that women may not experience menopause until they reach the age of 55, 60 or greater.
Female reproductive senescence begins before birth and continues throughout a lifetime, culminating with menopause, the state of depletion of ovarian follicles. The female reproductive axis is unique in that it reaches a senescent state when other organs in the body are generally healthy. On average, women reach menopause at 50 years of age whereas their life expectancy is over 80 years. Hence, the majority of women will spend, or are spending, more than one-third of their life in a post-menopausal state. Epidemiological studies show that late onset of menopause confers longevity and decelerates the appearance of much age-related morbidity, suggesting that delaying the onset of menopause will improve the health of aging women and therefore their quality of life. In this study, using animal models, we aim to develop an experimental procedure that will be conceptually applicable to delaying the natural onset of menopause in women. Once developed and successfully executed, this novel procedure may delay the onset of menopause by multiple years so that women may not experience menopause until they reach the age of 55, 60 or greater. 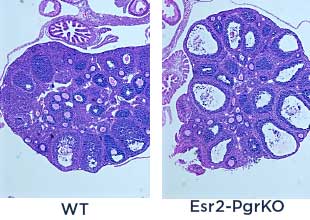
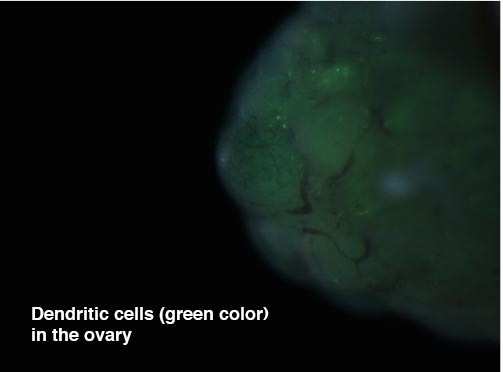
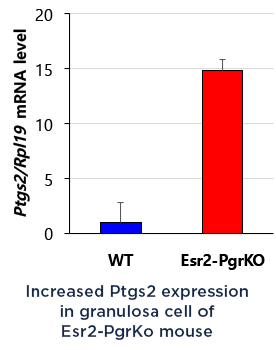 we found that PTGS2 expression was potentially attenuated by PGR in the granulosa cells. Therefore, we hypothesize that PGR activation suppresses cytokine productions, leukocyte recruiting and ovulatory inflammation via inhibiting PTGS2 expression. We will identify the inhibition mechanism of PGR on PTGS2 expression and anti-inflammatory role of PGR in the preovulatory ovary.
we found that PTGS2 expression was potentially attenuated by PGR in the granulosa cells. Therefore, we hypothesize that PGR activation suppresses cytokine productions, leukocyte recruiting and ovulatory inflammation via inhibiting PTGS2 expression. We will identify the inhibition mechanism of PGR on PTGS2 expression and anti-inflammatory role of PGR in the preovulatory ovary.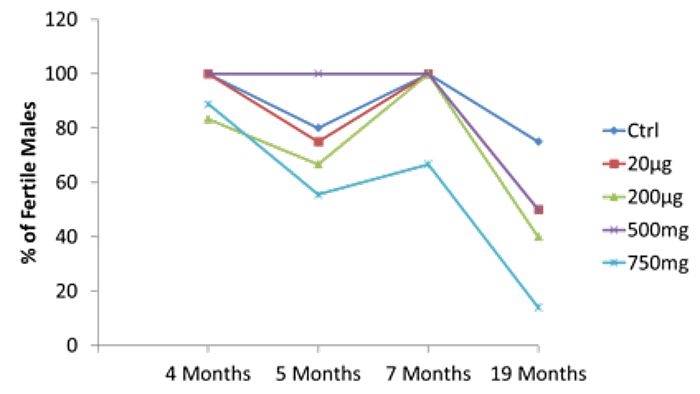
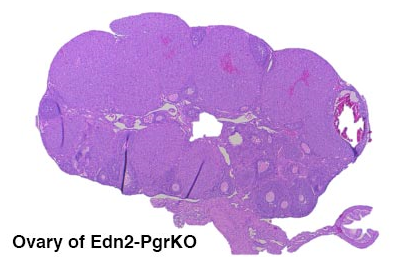 The focus of this study is to determine the functional role of progesterone receptor in regulating progesterone synthesis in the Corpus Luteum. We hypothesize that progesterone receptor is required for continued synthesis of progesterone during the luteal phase. For this study, we use transgenic mice that are selectively deficient of progesterone receptor in the luteal cells. To confirm the functioning role of the progesterone receptor, fertility, P4 concentration in the serum and the ovary are being currently assessed. Future studies include luteal cell cultures and determination of steroidogenic gene expression under the deficiency of progesterone receptor.
The focus of this study is to determine the functional role of progesterone receptor in regulating progesterone synthesis in the Corpus Luteum. We hypothesize that progesterone receptor is required for continued synthesis of progesterone during the luteal phase. For this study, we use transgenic mice that are selectively deficient of progesterone receptor in the luteal cells. To confirm the functioning role of the progesterone receptor, fertility, P4 concentration in the serum and the ovary are being currently assessed. Future studies include luteal cell cultures and determination of steroidogenic gene expression under the deficiency of progesterone receptor.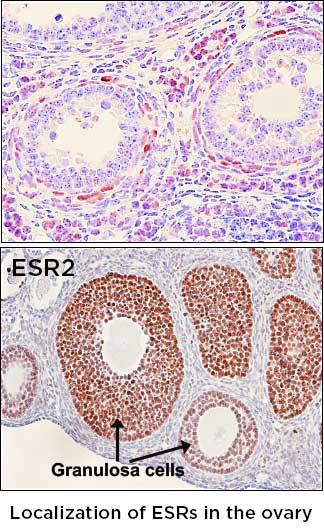 17β-estradiol (E2) is a steroid hormone that regulates a plethora of reproductive, metabolic, immunologic, cognitive, and skeletal functions. Two classical estrogen receptors, ESR1 (ERα) and ESR2 (ERβ) are responsible for the classical actions of E2 in mammalian species. In the ovary, ESR1 is localized to the surface epithelium and theca cells, whereas ESR2 is expressed in the granulosa cells.
17β-estradiol (E2) is a steroid hormone that regulates a plethora of reproductive, metabolic, immunologic, cognitive, and skeletal functions. Two classical estrogen receptors, ESR1 (ERα) and ESR2 (ERβ) are responsible for the classical actions of E2 in mammalian species. In the ovary, ESR1 is localized to the surface epithelium and theca cells, whereas ESR2 is expressed in the granulosa cells.