Information about the Saif Lab.
 The Saif Lab has two research thrusts: mechanics of living cells (biomechanics), and nanoscale materials (nanomechanics). For the former, our studies of cell mechanics are motivated by three primary reasons:
The Saif Lab has two research thrusts: mechanics of living cells (biomechanics), and nanoscale materials (nanomechanics). For the former, our studies of cell mechanics are motivated by three primary reasons:
- Understanding mechanotransduction: How cells transduce mechanical stimuli into biochemical processes and vice versa?
- Disease detection and prognosis: Is there a mechanical signature for a disease state in a cell?
- Biological machines: Can cell behavior be tuned by mechanical stimuli such that they self organize into functional units?
Our goals within these primary reasons are to explore questions such as: do memory and learning in animals depend on mechanical forces in neurons? Can cardiac cells synchronize their beating through long-range mechanical forces transmitted through the elastic media? Does the mechanical stiffness of cancer tumors play a role in initiating metastasis, and if so, how? What mechanical cues may lead to the emergence of biological machines from clusters of living cells?
As for the latter research thrust, our goal is to explore and understand the role of small size scale of material samples (such as thin films or nanowires) and their microstructures in determining their thermo-mechanical properties, the interaction between the macroscopic mechanisms of deformation and the interfaces at nanoscale, and the new mechanisms that interfaces may generate (structure-property relation, a fancier way of saying).
The underlying themes that link the two thrusts are the principles of mechanics and the processes that are relevant at a small scale. We are interested in the fundamental mechanisms that determine the processes. We use both experiment and theory to address our questions. In order to carry out exploratory experiments, we often develop our own micro and nano-machines and apparatus (microelectromechanical systems, or MEMS), and study their mechanics as well. A few contributions from our lab are summarized here.
Our Principal Investigator is Taher Saif.
Check out the tabs below or our website here to learn more.
Neuromechanics
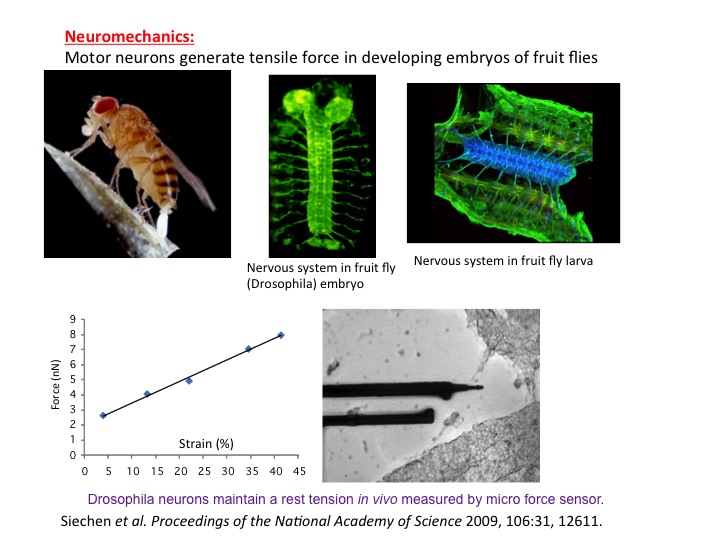 We have shown a potential link between mechanical tension in neurons, and memory and learning in animals. It has so far been believed that biochemical signaling is the basis for memory formation and learning process. Mechanical force in neurons has never been considered as a possible player. It is well understood today that memory and learning are mediated by neurotransmitters that are released from vesicles clustered at the synaptic terminal (endpoint of axon – a long arm of the neuron). As a synapse is used more frequently, its neurotransmission efficiency increases, partly because of increased vesicle clustering in the synapse, i.e., the synapse remembers its past usage, which offers the basis for understanding memory. We (together with Chiba lab, University of Maimi) conducted in vivo experiments on embryonic Drosophila (fruit fly) nervous system and showed that vesicle clustering at the neuromuscular synapse depends on mechanical tension within the axons. Vesicle clustering vanishes upon severing the axon from the cell body but is restored when mechanical tension is applied to the severed end of the axon. Clustering increases when intact axons are stretched mechanically. Using nanomechanical force sensors that touch single neurons of the embryos, we found that embryonic axons that have formed neuromuscular junctions maintain a rest tension of about one nanonewton, which appears to be essential for neurotransmission. The study thus adds a new paradigm in the understanding of memory and learning.
We have shown a potential link between mechanical tension in neurons, and memory and learning in animals. It has so far been believed that biochemical signaling is the basis for memory formation and learning process. Mechanical force in neurons has never been considered as a possible player. It is well understood today that memory and learning are mediated by neurotransmitters that are released from vesicles clustered at the synaptic terminal (endpoint of axon – a long arm of the neuron). As a synapse is used more frequently, its neurotransmission efficiency increases, partly because of increased vesicle clustering in the synapse, i.e., the synapse remembers its past usage, which offers the basis for understanding memory. We (together with Chiba lab, University of Maimi) conducted in vivo experiments on embryonic Drosophila (fruit fly) nervous system and showed that vesicle clustering at the neuromuscular synapse depends on mechanical tension within the axons. Vesicle clustering vanishes upon severing the axon from the cell body but is restored when mechanical tension is applied to the severed end of the axon. Clustering increases when intact axons are stretched mechanically. Using nanomechanical force sensors that touch single neurons of the embryos, we found that embryonic axons that have formed neuromuscular junctions maintain a rest tension of about one nanonewton, which appears to be essential for neurotransmission. The study thus adds a new paradigm in the understanding of memory and learning.
Currently, we are addressing the following questions:
- What is the mechanism of force generation in embryonic fly axons?
- What is the role of axonal tension in postsynaptic excitation?
- How does tension in axons modulate short and long term potentiation?
- What is the role of tension in axonal transport?
We are using embryonic fruit flies, adult crayfish, and rat brain slices to address these questions.
Biological machines
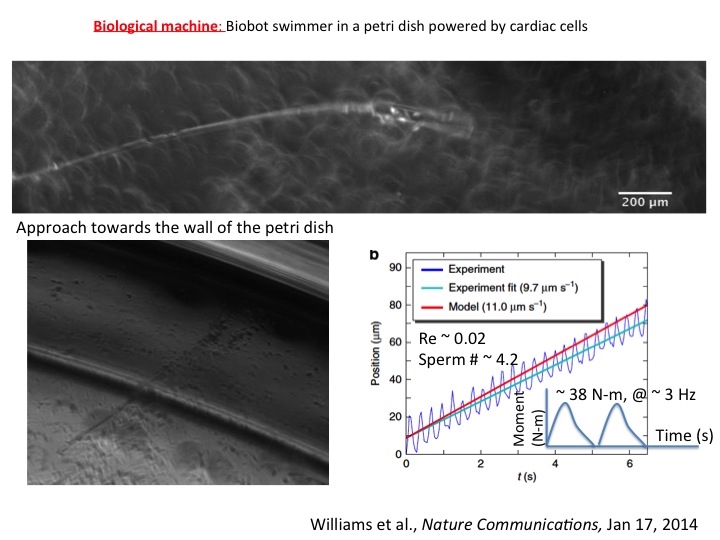 We have shown that cardiomyocytes plated on a flexible string can communicate with each other, self-organize, and emerge into a cell cluster that beats synchronously. This results in a swimming artificial flagellum. Its dynamics of well predicted by our theoretical model based on slender body hydrodynamics. This is the first demonstration of a small-scale swimmer that propels autonomously in a viscous environment.
We have shown that cardiomyocytes plated on a flexible string can communicate with each other, self-organize, and emerge into a cell cluster that beats synchronously. This results in a swimming artificial flagellum. Its dynamics of well predicted by our theoretical model based on slender body hydrodynamics. This is the first demonstration of a small-scale swimmer that propels autonomously in a viscous environment.
Currently, we are addressing the following questions:
- What is the underlying mechanism of synchrony between cardiomyocytes that are separated from one another?
- Is swimming possible by muscle cells that can be actuated by light so that we can achieve controlled actuation?
- Can we develop swimmers using neurons and muscle cells so that the swimmers may have intelligence and memory? Such swimmers may be used for targeted drug delivery in vivo.
Mechanics of Cancer Metastasis
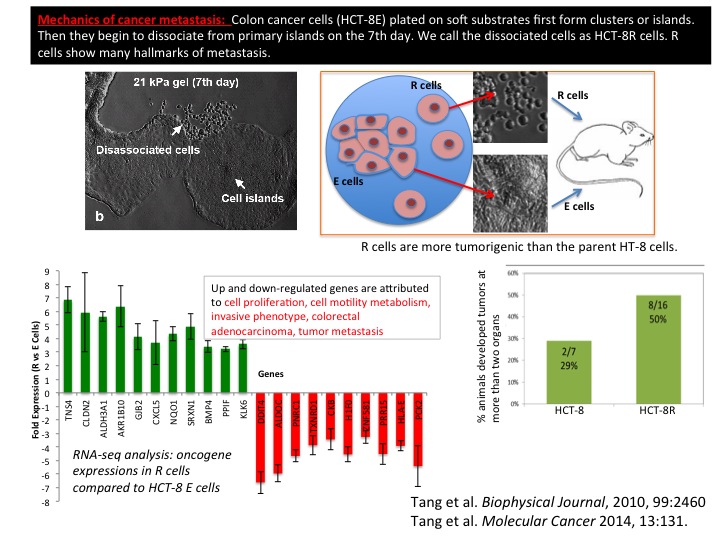 We have shown that cancer cells (human colon carcinoma, HCT-8) can exhibit metastasis like phenotype in vitro induced solely by an appropriate mechanical softness of the microenvironment. This rather remarkable phenotype is observed when HCT-8 cells are cultured on gels with intermediate-stiffness (physiologically relevant 21 kPa- 47 kPa), but not on very soft (1 kPa) and hard (3.6 GPa) substrates. The cell-cell adhesion molecule E-Cadherin, a metastasis hallmark, decreases 3-5 fold on cell membranes in concert with disassociation. Both specific and non-specific cell adhesion (measured by a Bio-MEMS force sensor) decrease once the cells have disassociated. After re-culturing the disassociated cells on fresh substrates, they retain the disassociated phenotype regardless of substrate stiffness. These results indicate that during culture on the appropriate mechanical microenvironment, HCT-8 cells undergo a stable cell-state transition with increased in vitro metastasis-like characteristics as compared to parent cells grown on standard, stiff tissue culture dishes. When implanted in mouse spleen, the dissociated cells show higher tumorigenicity compared to the parent cells. This novel finding suggests the onset of metastasis may be fundamentally linked to the mechanical microenvironment of the tumor.
We have shown that cancer cells (human colon carcinoma, HCT-8) can exhibit metastasis like phenotype in vitro induced solely by an appropriate mechanical softness of the microenvironment. This rather remarkable phenotype is observed when HCT-8 cells are cultured on gels with intermediate-stiffness (physiologically relevant 21 kPa- 47 kPa), but not on very soft (1 kPa) and hard (3.6 GPa) substrates. The cell-cell adhesion molecule E-Cadherin, a metastasis hallmark, decreases 3-5 fold on cell membranes in concert with disassociation. Both specific and non-specific cell adhesion (measured by a Bio-MEMS force sensor) decrease once the cells have disassociated. After re-culturing the disassociated cells on fresh substrates, they retain the disassociated phenotype regardless of substrate stiffness. These results indicate that during culture on the appropriate mechanical microenvironment, HCT-8 cells undergo a stable cell-state transition with increased in vitro metastasis-like characteristics as compared to parent cells grown on standard, stiff tissue culture dishes. When implanted in mouse spleen, the dissociated cells show higher tumorigenicity compared to the parent cells. This novel finding suggests the onset of metastasis may be fundamentally linked to the mechanical microenvironment of the tumor.
Currently, we are addressing the following questions:
- What is the role of intracellular forces in transforming the parent HCT-8 cells into metastatic ones?
- Can cell forces be used as a marker for drug screening?
- Is there a mechanical signature in metastatic colon cancer cells from human patients? Can such signature(s) be used for cancer prognosis?
These questions are explored in collaboration with Carle Foundation Hospital, Presence Hospital, and Mayo Clinic.
Effect of size on BDT (brittle to ductile transition) temperature in single crystal silicon
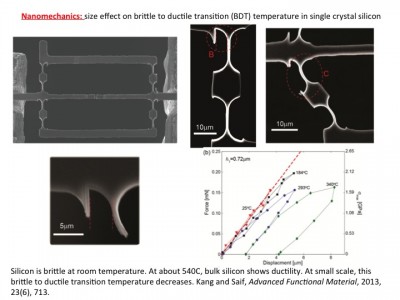 Silicon-based micro- and nanometer-scale devices operating at various temperatures are ubiquitous today. However, thermo-mechanical properties of silicon at the small scale and their underlying mechanisms remain elusive. The brittle-to-ductile transition (BDT) is one such property relevant to these devices. Materials can be brittle or ductile depending on temperature. The BDT occurs over a small temperature range. For bulk silicon, the BDT is about 545°C. It is speculated that the BDT temperature of silicon may decrease with size at the nanoscale. However, recent experimental and computational studies have provided inconclusive evidence, and are often contradictory. Potential reasons for the controversy might originate from the lack of an in situ methodology that allows variation of both temperature and sample size. We resolved this controversy by carrying out in situ thermo-mechanical bending tests on single crystal silicon samples with concurrent control of these two key parameters. We showed unambiguously that the BDT temperature reduces with sample size. For example, the BDT temperature decreases to 293°C for a sample size of 720 nm. We developed a mechanism-based model to interpret the experimental observations.
Silicon-based micro- and nanometer-scale devices operating at various temperatures are ubiquitous today. However, thermo-mechanical properties of silicon at the small scale and their underlying mechanisms remain elusive. The brittle-to-ductile transition (BDT) is one such property relevant to these devices. Materials can be brittle or ductile depending on temperature. The BDT occurs over a small temperature range. For bulk silicon, the BDT is about 545°C. It is speculated that the BDT temperature of silicon may decrease with size at the nanoscale. However, recent experimental and computational studies have provided inconclusive evidence, and are often contradictory. Potential reasons for the controversy might originate from the lack of an in situ methodology that allows variation of both temperature and sample size. We resolved this controversy by carrying out in situ thermo-mechanical bending tests on single crystal silicon samples with concurrent control of these two key parameters. We showed unambiguously that the BDT temperature reduces with sample size. For example, the BDT temperature decreases to 293°C for a sample size of 720 nm. We developed a mechanism-based model to interpret the experimental observations.
Currently, we are addressing the following questions:
- What is the mechanism by which silicon reduces the BDT temperature with decreasing size?
- What is the role of size in determining the yield strength of silicon under bending? What is the underlying mechanism?
We are addressing these questions in collaboration with the University of Vienna and Max Planck Institute, Düsseldorf, Germany.