Information about the Rhodes Laboratory.
 The Rhodes Laboratory is broadly interested in how genes and environment shape voluntary behavior. Most of our work involves performing behavioral experiments with mice and fish to answer basic questions about how lifestyle factors such as diet, drugs of abuse, social environment, and exercise affect the brain and behavior. Our approach combines behavioral, genetic, molecular, and histological techniques and involves extensive collaborations with different units on campus and outside campus including engineering, kinesiology, and nutrition.
The Rhodes Laboratory is broadly interested in how genes and environment shape voluntary behavior. Most of our work involves performing behavioral experiments with mice and fish to answer basic questions about how lifestyle factors such as diet, drugs of abuse, social environment, and exercise affect the brain and behavior. Our approach combines behavioral, genetic, molecular, and histological techniques and involves extensive collaborations with different units on campus and outside campus including engineering, kinesiology, and nutrition.
Our mice and fish are in two separate projects at the moment. Current mouse projects focus on uncovering origins of exercise-brain interactions, with two separate lines of research: one on muscle-brain communication and the other on brain activation associated with physical exertion. We also have an ongoing project exploring the impact of natural versus synthetic vitamin E on brain development. Meanwhile, our fish work currently has two main objectives which fill important gaps in our understanding of sex differences in the brain and the regulation of parental care. The first is uncovering the neuroendocrine mechanisms involved in the active feminization of the brain induced by social dominance and the latter on fathering behavior. The unique life history of the fish we are studying allows us to explore questions which are much more difficult if not impossible to study in mammals, but presumably, follow similar biological processes because of evolutionary ancestry.
Our Principal Investigator is Justin Rhodes.
Want to learn more about our lab? Click here.
Nutrition, Learning, and Memory
 The Rhodes Lab houses the Mouse Cognition Core Facility for the Center for Nutrition, Learning, and Memory, a privately funded center which supports multiple projects exploring micronutrient impacts on cognitive performance in humans and animal models. The Core Facility was created to understand how nutrition can optimize brain health across the lifespan along with finding cognitive and behavioral paradigms that will elucidate the effect various nutritional compounds and supplements may have on the brain and behavior. The facility collaborates with both University of Illinois scientists as well as scientists from Abbott Nutrition. Additionally, our lab is also interested in how products of metabolism signal the brain to influence behavioral outcomes such as physical activity, cognitive performance, and food intake.
The Rhodes Lab houses the Mouse Cognition Core Facility for the Center for Nutrition, Learning, and Memory, a privately funded center which supports multiple projects exploring micronutrient impacts on cognitive performance in humans and animal models. The Core Facility was created to understand how nutrition can optimize brain health across the lifespan along with finding cognitive and behavioral paradigms that will elucidate the effect various nutritional compounds and supplements may have on the brain and behavior. The facility collaborates with both University of Illinois scientists as well as scientists from Abbott Nutrition. Additionally, our lab is also interested in how products of metabolism signal the brain to influence behavioral outcomes such as physical activity, cognitive performance, and food intake.
The projects are led by interdisciplinary teams across multiple laboratories on campus and the nation. Our core provides the staff, equipment, and expertise for performing behavioral testing in mice including multiple tests of learning and memory. In addition, the core facility provides support for performing molecular biology (RNA-seq analysis, rt-PCR, ELISA, mouse genotyping) and for collecting and analyzing neuroanatomical measurements in mice, mostly using immunohistochemistry and stereology. Collaborative projects supported by the core include developing a mouse model of chemobrain for nutritional intervention, projects exploring interactions between exercise, dietary fiber and other micronutrients on behavioral performance in mice, and the role of HMB in promoting enhanced cognitive function through muscle-brain interactions. We have also directly supported projects with Abbott Nutrition scientists exploring the role of natural versus synthetic vitamin E on neurological development in a few different mouse models. Finally, with Abbott Nutrition scientists, we are trying to find combinations of micronutrients that reliably enhance behavioral performance in aged mice.
Origins of Exercise-Brain Interactions
 It is now established that incorporating regular exercise into the life routine is crucial for maintaining cognitive health throughout the lifespan, however, the mechanisms for the pro-cognitive effects of exercise are not well understood. Elucidating and unequivocally establishing these mechanisms holds the key to discover novel and more efficient ways to maintain, promote and improve cognitive performance, perhaps even in the absence of physical activity. We and many others have documented numerous long-term changes in the central nervous system (CNS) from exercise training which likely support enhanced cognition. However, the acute effects of exercise which ultimately must cause the long-term CNS adaptations are not known. Moreover, it is not clear where the acute signals come from, the periphery (e.g., muscles, blood pressure) or within the brain itself. We believe it is crucial to identify those specific acute events associated with exercise or physical exertion that when repeated contribute to the chronic neurological changes that have been so well documented and presumably related to the cognitive enhancement. This is because if we seek to replicate exercise’s pro-cognitive influence, then it likely will be necessary to recapitulate the acute events and repeat them to cause the desired long-term cognitive benefits, as occurs with exercise training.
It is now established that incorporating regular exercise into the life routine is crucial for maintaining cognitive health throughout the lifespan, however, the mechanisms for the pro-cognitive effects of exercise are not well understood. Elucidating and unequivocally establishing these mechanisms holds the key to discover novel and more efficient ways to maintain, promote and improve cognitive performance, perhaps even in the absence of physical activity. We and many others have documented numerous long-term changes in the central nervous system (CNS) from exercise training which likely support enhanced cognition. However, the acute effects of exercise which ultimately must cause the long-term CNS adaptations are not known. Moreover, it is not clear where the acute signals come from, the periphery (e.g., muscles, blood pressure) or within the brain itself. We believe it is crucial to identify those specific acute events associated with exercise or physical exertion that when repeated contribute to the chronic neurological changes that have been so well documented and presumably related to the cognitive enhancement. This is because if we seek to replicate exercise’s pro-cognitive influence, then it likely will be necessary to recapitulate the acute events and repeat them to cause the desired long-term cognitive benefits, as occurs with exercise training.
We are currently working to put together a team of scientists, to focus on this question and compete for an NIH Program Project or Center on origins of exercise effects on the brain. The team would require experts from such different disciplines as kinesiology, psychology, behavioral neuroscience, chemical engineering, and biochemistry. One line of research would test the hypothesis that the effects of exercise on enhanced survival and differentiation of new neurons in the hippocampus is due to contraction of the peripheral muscles engaged during the physical activity. Another line will test the hypothesis that the origin of exercise-induced neurogenesis comes from the repeated acute activation of the dentate gyrus that occurs during high-intensity movements.
Socially Influenced Sex Change In Anemonefishes
 Clownfishes typically live most of their life on or in very close proximity to a sea anemone in small groups from 3 up to 6 or 7 depending on the size of the anemone. In these groups, the largest is usually the reproductive female, the second-largest, the male and the smaller individuals are undifferentiated. If the female is removed, the male will transform into a female in a matter of weeks. Immediately, within the first few minutes, the male will begin to display female-typical behavior, e.g., court the other smaller fish. Within a few weeks, the testes of the male will have been absorbed, and ovaries will replace them. In addition, the size of the fish will increase, and the genitalia will change shape to allow for the passage of eggs instead of sperm through the genital duct. At the same time, the largest of the undifferentiated fish will transform into a male following a similar time course.
Clownfishes typically live most of their life on or in very close proximity to a sea anemone in small groups from 3 up to 6 or 7 depending on the size of the anemone. In these groups, the largest is usually the reproductive female, the second-largest, the male and the smaller individuals are undifferentiated. If the female is removed, the male will transform into a female in a matter of weeks. Immediately, within the first few minutes, the male will begin to display female-typical behavior, e.g., court the other smaller fish. Within a few weeks, the testes of the male will have been absorbed, and ovaries will replace them. In addition, the size of the fish will increase, and the genitalia will change shape to allow for the passage of eggs instead of sperm through the genital duct. At the same time, the largest of the undifferentiated fish will transform into a male following a similar time course.
The phenomenon of neuroplasticity is perhaps no better exemplified than in the anemonefishes and many other coral reef marine fishes which change sex depending on the outcome of territorial contests. For the past 5 years, we have been maintaining a colony of Amphiprion ocellaris, a species of anemonefish easily kept and bred in the laboratory, to study how the brain, pituitary, and gonadal tissues of these fishes coordinate a complete and seamless sex transformation. So far we discovered that arginine vasotocin signaling at V1a receptors is necessary for males to exert aggression and dominance in dyadic contests which precede sex change in our species. Moreover, we discovered that males are the primary care-givers of the eggs and that isotocin and arginine vasotocin play opposing roles in the regulation of paternal care.
Wireless Optogenetics For Manipulating Behavior of Mice
 Despite decades of intense research since the discovery that new neurons are continuously generated in the adult mammalian brain, their function remains a mystery. Determining the role of neurogenesis in healthy and diseased brains has broad implications for our understanding of learning, memory, behavior, and regenerative medicine. We are taking advantage of recent advances in optogenetic technology to design tools for studying new neurons in vivo. By combining optogenetics (which uses light to control genetically modified cells) with our lab’s expertise in behavior, we are making progress towards solving one of the biggest mysteries in neuroscience.
Despite decades of intense research since the discovery that new neurons are continuously generated in the adult mammalian brain, their function remains a mystery. Determining the role of neurogenesis in healthy and diseased brains has broad implications for our understanding of learning, memory, behavior, and regenerative medicine. We are taking advantage of recent advances in optogenetic technology to design tools for studying new neurons in vivo. By combining optogenetics (which uses light to control genetically modified cells) with our lab’s expertise in behavior, we are making progress towards solving one of the biggest mysteries in neuroscience.
The ability to label specific populations of neurons with excitatory and inhibitory opsins and then use light to control activation of these selected neurons has tremendous potential for helping us understand how neural circuits control behavior. However, a major limitation has been the bulky headgear or fiber optic cables that must be mounted into the head to provide light to the neural tissue, and which can severely limit the movement of the animal. To overcome this obstacle we developed an intensive collaboration with the John Rogers’ group (Professor, Material Sciences) on campus to develop wireless, μLEDs that could be implanted into various different brain regions and illuminated remotely as the animal freely moves through behavioral apparatuses. After more than 2 years of iterative research between our laboratories, beta testing devices, and developing various transgenic lines of mice for optogenetic manipulations, we now have a few different working applications. These include transgenic lines of mice that enable the labeling of cohorts of new neurons born during a period of exercise with green fluorescent protein and archeorhodopsin. These new neurons can then be instantaneously inactivated while the animals are performing a specific behavior through wireless illumination of the implanted μLEDs from the Rogers’ group. Using this technology we have collected preliminary data that when mice are trained to associate a context with a mild electric shock, their memory of the association is partially dependent on the function of the new neurons labeled during the exercise period. The result has also provided a validation of the optoelectronic device for inactivating neurons and thereby affecting behavior.
John now has a company, Neurolux, which sells the devices.
Mice Selectively Bred for Increased Voluntary Wheel Running to Study Motivation for Exercise
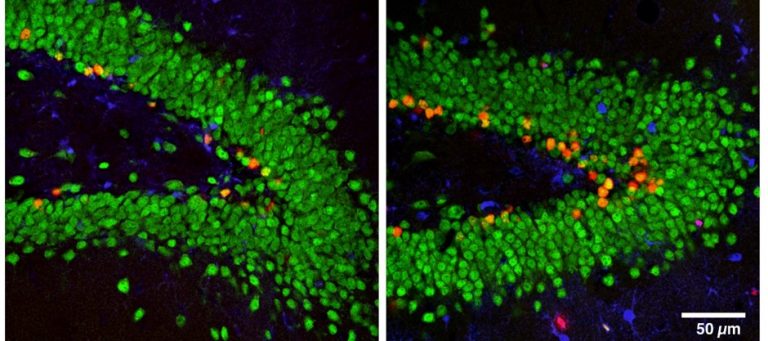 A large literature has established the critical importance of aerobic exercise for maintaining physical and mental health throughout the lifespan, yet average daily levels of physical activity continue to decline in western society. The major obstacle preventing the global therapeutic application of exercise is that for many people, the desire to exercise is low, and appears to be declining. Moreover, it is not clear how to increase motivation for exercise. Physical exercise can be rewarding and addictive in certain individuals, suggesting that motivation for physical activity can be increased. However, relatively few studies have investigated the neurobiology of increased motivation for exercise and our understanding of how to neurologically increase the desire to exercise is rudimentary at best.
A large literature has established the critical importance of aerobic exercise for maintaining physical and mental health throughout the lifespan, yet average daily levels of physical activity continue to decline in western society. The major obstacle preventing the global therapeutic application of exercise is that for many people, the desire to exercise is low, and appears to be declining. Moreover, it is not clear how to increase motivation for exercise. Physical exercise can be rewarding and addictive in certain individuals, suggesting that motivation for physical activity can be increased. However, relatively few studies have investigated the neurobiology of increased motivation for exercise and our understanding of how to neurologically increase the desire to exercise is rudimentary at best.
We propose to use the replicate lines of mice selectively bred for increased voluntary wheel-running behavior to find genes and resulting neurophysiological traits which predispose high motivation for running. We have several specific hypotheses we would like to test based on results of a recent RNA-sequencing study of the striatum. Our approach would be to use transgenic mouse technology and local infusion of RNA interfering molecules to knock down or increase the expression of the targeted genes. We also have plans to test the efficacy of novel small molecules to increase motivation for exercise in laboratory strains displaying low levels of activity. A recent bioinformatics analysis of the RNA-seq data identified a shortlist of small molecules potentially capable of partially replicating the striatal gene expression profile of high runners and thereby increasing motivation for running.
Mice Selectively Bred for Increased Home Cage Activity As a Model For ADHD
 We have developed a method, using sophisticated video tracking software from CleverSysems Inc., to measure physical activity in home cages 24 hours a day 7 days a week in hundreds of mice each generation. We are currently breeding lines of mice for high levels of cage activity while maintaining other un-selected lines to serve as controls. The goal is to identify how genes influence behavior at multiple levels of biological organization from the genes to the development and function of a nervous system.
We have developed a method, using sophisticated video tracking software from CleverSysems Inc., to measure physical activity in home cages 24 hours a day 7 days a week in hundreds of mice each generation. We are currently breeding lines of mice for high levels of cage activity while maintaining other un-selected lines to serve as controls. The goal is to identify how genes influence behavior at multiple levels of biological organization from the genes to the development and function of a nervous system.
In addition to continuing to work on the lines of mice selectively bred for increased voluntary wheel running, we developed two lines derived from the highly genetically variable 8-way Collaborative (or Diversity) Cross. One line is selectively bred for increased home cage activity each generation using video tracking in cages without wheels to measure horizontal movement, and one is randomly bred to serve as the control. The lines are currently in generation 19, approximately 7 years of work.
Recently, we discovered that mice from the high-active line display motor impulsivity suggesting that hyperactivity and impulsivity are inevitably entangled traits influenced by similar suites of genes in our lines. Further, we established that the hyperactivity and impulsivity are ameliorated with amphetamines given at similar doses used to treat Attention Deficit Hyperactivity Disorder (ADHD). Our current proposal is to use the newly validated model to find the genes implicated in hyperactivity/impulsivity and as a platform for ADHD medication development.