Information about the Computational Structural Biology and Molecular Biophysics Group.
 The Computational Structural Biology and Molecular Biophysics Group seeks to characterize the structural and dynamical properties of macromolecular systems that furnish their biological function. We employ molecular modeling and simulation technologies to investigate various biomolecular systems at an atomic resolution and to reveal molecular events and processes that are fundamental to their function. Given the unparalleled temporal and spatial resolutions offered by these technologies, the knowledge acquired by these studies is highly complementary to that derived from experimental approaches, thus, allowing one to develop a more complete picture of the mechanism of function of biomolecular systems. In short, we strive to understand how biomolecules work!
The Computational Structural Biology and Molecular Biophysics Group seeks to characterize the structural and dynamical properties of macromolecular systems that furnish their biological function. We employ molecular modeling and simulation technologies to investigate various biomolecular systems at an atomic resolution and to reveal molecular events and processes that are fundamental to their function. Given the unparalleled temporal and spatial resolutions offered by these technologies, the knowledge acquired by these studies is highly complementary to that derived from experimental approaches, thus, allowing one to develop a more complete picture of the mechanism of function of biomolecular systems. In short, we strive to understand how biomolecules work!
Our Group Leader is Emad Tajkorshid, J.W. Hastings Endowed Chair of Biochemistry and Director of NIH Center for Macromolecular Modeling and Bioinformatics.
Check out our website here to learn more!
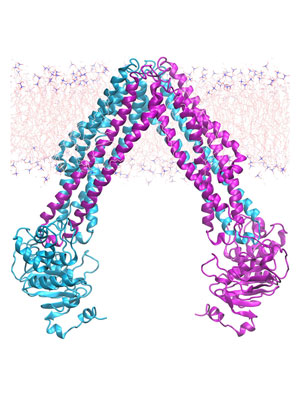
Mechanistic Studies of Membrane Transporters
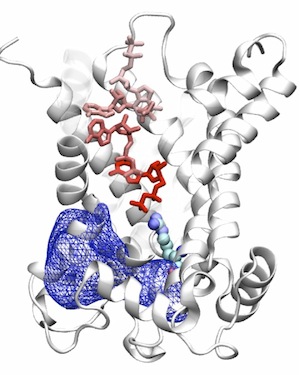 Mitochondrial Nucleotide Exchange by AAC
Mitochondrial Nucleotide Exchange by AAC
The ADP/ATP carrier (AAC) is the only structurally known member of the mitochondrial carrier family (MCF), which is responsible for the exchange of ATP and ADP across the inner membrane of mitochondria. We employ computational and simulation methodologies in order to study the dynamics of this carrier, to characterize the binding site of the substrate, and to develop a model for major structurally unknown sates.
Alternating Access Mechanism in Major Facilitator Superfamily
 GlpT, glycerol-3-phosphate:phosphate antiporter, is among the few structurally known members of Major Facilitator Superfamily. Alternating -access mechanism, which asserts that the single binding site of the transporter is only accessible from either side of the membrane simultaneously, has been proposed. Our simulations captured spontaneous substrate recruitment from the opening of the lumen of GlpT and conformational changes induced by substrate binding that are consistent with the alternating-access mechanism.
GlpT, glycerol-3-phosphate:phosphate antiporter, is among the few structurally known members of Major Facilitator Superfamily. Alternating -access mechanism, which asserts that the single binding site of the transporter is only accessible from either side of the membrane simultaneously, has been proposed. Our simulations captured spontaneous substrate recruitment from the opening of the lumen of GlpT and conformational changes induced by substrate binding that are consistent with the alternating-access mechanism.