Information about the Clockworks Lab.
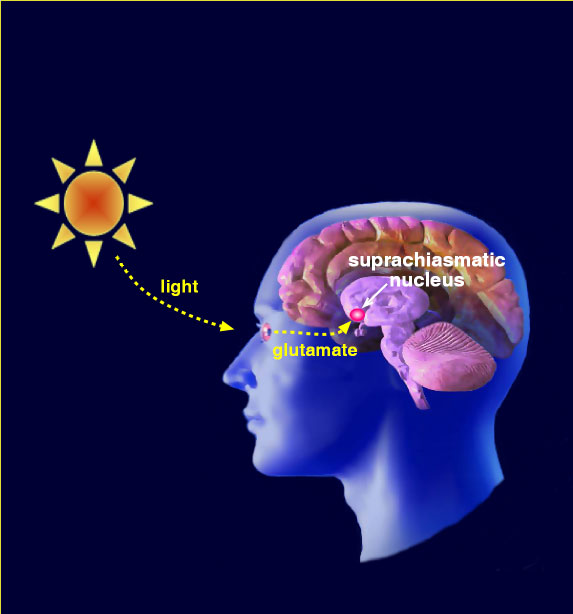 The Clockworks Lab seeks answers as to what why do current biological functions and behaviors occur (birds singing in the morning vs frogs calling at night) or at least, more likely to occur (heart attacks before dawn and asthmatic attacks after sunset) at a particular time of day or a season (feeling lethargic and depressed during the winter vs feeling energetic and alert during the summer). The answer lies in understanding the central role of the brain's clock in organizing our body functions around the major variable in the external world, the daily cycle of darkness and light. This circadian clock, located in the suprachiasmatic nucleus (SCN) of the brain, whose cellular processes mark the passage of time in near 24-hr cycles, is a fundamental life component. Circadian clocks impose temporal order on cells, tissues, and organs throughout the body, modulating body processes over the day-night cycle. Our broad research objective is to understand how biological timing systems control integrative brain functions.
The Clockworks Lab seeks answers as to what why do current biological functions and behaviors occur (birds singing in the morning vs frogs calling at night) or at least, more likely to occur (heart attacks before dawn and asthmatic attacks after sunset) at a particular time of day or a season (feeling lethargic and depressed during the winter vs feeling energetic and alert during the summer). The answer lies in understanding the central role of the brain's clock in organizing our body functions around the major variable in the external world, the daily cycle of darkness and light. This circadian clock, located in the suprachiasmatic nucleus (SCN) of the brain, whose cellular processes mark the passage of time in near 24-hr cycles, is a fundamental life component. Circadian clocks impose temporal order on cells, tissues, and organs throughout the body, modulating body processes over the day-night cycle. Our broad research objective is to understand how biological timing systems control integrative brain functions.
Our research has important applications: Malfunctioning of the brain's circadian clock results in disorders in brain and organ function, which manifest themselves as clinical disorders of sleep, movement and neural degeneration, such as in Alzheimer's and Parkinson's diseases. The breadth of our systems-based analysis is generating insights into mechanisms that synchronize people to day and night, which is of proven importance to good health and disease-resistance. Outcomes will enhance understanding of substrates that generate long-term neural changes, with broad relevance for public health and disease prevention. They will enable strategies for ameliorating sleep, autonomic, degenerative, movement and cognitive disorders.
In addition, we are engaged in interdisciplinary research with Jonathan Sweedler to build upon campus excellence in molecular and cellular biology, nano-scale analytical chemistry and bioengineering. Our goal is to discover novel insights, solutions, and applications for neural repair and restoration of function through targeting critical molecules and processes that construct micro-networks during the normal wiring of the nervous system.
Our Principal Investigator is Martha Gillette.
Click here to learn more at our website.
Stimulus-Transcription Coupling
 A central unsolved question in biology is: What coordinates an organism’s circadian clocks? Loss of coordination between the central circadian clock in the brain, the suprachiasmatic nucleus (SCN), and circadian clocks in other cells and tissues has been implicated in systems pathologies that lead to sleep disorders.
A central unsolved question in biology is: What coordinates an organism’s circadian clocks? Loss of coordination between the central circadian clock in the brain, the suprachiasmatic nucleus (SCN), and circadian clocks in other cells and tissues has been implicated in systems pathologies that lead to sleep disorders.
The goal of this discovery proposal is to identify peptides that couple the SCN and glial circadian clocks. Studies of peripheral tissues cultured in isolation have revealed that in the SCN’s absence, cellular rhythms continue but phase and period properties change in diverse tissues, including brain, liver, lung, muscle, kidney, tail and spleen. With decoupling, the various tissue functions lose temporal coherence as well as appropriate alignment to the daily cycle of sleep and wakefulness. Little is known about what couples an organism’s circadian clocks, except that diffusible factors are sufficient to entrain many. Discovering coupling factors that communicate time-of-day from SCN to other circadian clocks in brain and body has proven difficult.
We propose a study applying advanced analytical peptidomics techniques on a micrometer scale coupled with functional determinations of the ability of peptides to restore clock-to-clock coordination, an innovative approach. We aim to:
- Define and characterize the induction of SCN-driven synchronization of glial rhythms
- Identify candidate coupling peptides by peptidomics analysis
- Initiate evaluating the necessity/sufficiency of candidate peptides for coupling glial clocks.
Loss of synchrony among internal clocks is maladaptive for health and longevity. There are no current approaches to better synchronize or enhance coupling of the internal clocks. Identifying signals that effectively couple circadian rhythms will have major value in the treatment of metabolic syndrome, obesity, cardiovascular stress, and physiological decline with aging, all of which manifest with disordered sleep patterns that affect more than 10 million Americans each year. However, to realize the therapeutic potential, signals by which the SCN engages other circadian clocks must be identified and placed in a temporal context
Molecular Gating of Signal Transduction
 The dynamic assembly and disassembly of the actin cytoskeleton underlies diverse cellular processes, including cell division, developmental polarity and intracellular transport. These changes can be local or global, transforming cell state. Extracellular signals mediate experience-induced changes of actin dynamics within synaptic microdomains of neurons. Recent evidence suggests that actin dynamics of the cell body, distinct from those in the synapse, also may be necessary for neurons to sense and respond to extracellular stimuli. We predict that this process contributes to the plasticity of behavior by altering transcription.
The dynamic assembly and disassembly of the actin cytoskeleton underlies diverse cellular processes, including cell division, developmental polarity and intracellular transport. These changes can be local or global, transforming cell state. Extracellular signals mediate experience-induced changes of actin dynamics within synaptic microdomains of neurons. Recent evidence suggests that actin dynamics of the cell body, distinct from those in the synapse, also may be necessary for neurons to sense and respond to extracellular stimuli. We predict that this process contributes to the plasticity of behavior by altering transcription.
Our overarching goal is to understand how experience signals long-term state changes in neurons that, in turn, change behavior. We hypothesize that glutamatergic neurotransmission changes actin organization, and this is permissive for transcriptional activation. We will test this hypothesis in the suprachiasmatic nucleus (SCN), a brain site with established molecular substrates necessary for temporal organization of behavior. The SCN is a cell-based, ~24-h clock driven by spatial and temporal oscillations that regulate transcription. Specifically, we hypothesize that signaling cascades initiated by glutamate engage the actin cytoskeleton of SCN cells, changing the localization of key transcriptional regulators that alter clock state.
We will examine the nature and necessity of such changes in actin and their effects on transcriptional activation of clock genes. We will evaluate these mechanisms in rat and mouse models: cell cultures, brain slices, and behaving animals. Specific aims will:
- Characterize and localize stimulus-induced changes in actin
- Find the role of actin changes in clock function and behavior
- Determine the role of actin changes in regulating transcription.
We will use cell biological methods, dynamic imaging, biochemistry, neurobiological measures, and behavioral analyses. The breadth of this systems-based analysis will generate insights into how experience is transformed into long-lasting modification in brain state and behavior. This will enhance the understanding of substrates of long-lasting neural state change, with broad relevance for public health and disease prevention. Dysfunctions in the actin system cause severe neurological disorders, including those of cognition, neurodegeneration, movement and autonomic control. Sleep disorders, learning/memory impairments, drug-addiction, and aging will be direct beneficiaries.
Nano-Scale Processes of Dendrogenesis
Proper wiring of the nervous system requires the interplay of intrinsic and extrinsic signals that shape neurite development, architecture, and function. Whereas axonal development is relatively well understood, less is known of the forces that shape dendrites, especially the nano-scale filopodia that decorate developing dendritic shafts. What factors influence dendritic filopodia during wiring of brain circuits? Do filopodia contribute to the formation of dendritic spines, sites of synaptic information processing and plasticity?
We hypothesize that chemical cues in substrate-bound gradients instruct dendrite morphogenesis and maturation via nanometer-scale changes that transform collateral filopodia into spines. We will build upon our recent success in culturing hippocampal neurons from early post-natal rats at very low densities in refined microfluidic environments. Centered in neuroscience, this R21 proposal bridges with materials science to create and exploit complex gradient chemical fields—ones embedding nanometer-scale design rules and capable of imprinting the physical environments of neurons in culture with specific immobilized and diffusive factors.
These experimental competencies are provided by state-of-the-art microfluidic systems that exploit a variety of physical behaviors to actuate programmed chemo-temporal profiles within the device. Specific aims are to:
- Characterize collateral filopodial behavior in response to 2D surface gradients of bioactive molecules
- Build upon these findings to construct 3D gradient environments that encourage filopodial differentiation and enable responses to diffusive stimuli.
Models are hippocampal neurons of early post-natal rat and EGFP-actin transgenic mouse. We seek to discover novel insights, solutions, and applications that impact mental health, neural repair, and restoration of function. The intransigence of brain disorders and damage to treatment is of rising concern as many incurable conditions (schizophrenia, depression, Parkinson’s and Alzheimer’s disease) have huge economic costs and will increase with the aging of our population.