Information about the Chung Lab.
 The Chung lab seeks to have a better understanding of the pathogenesis of epilepsy. Understanding the pathogenesis is critical to developing novel therapeutic interventions and early diagnostics for epilepsy. “How does a neuron change itself to produce excessive electrical signals (intrinsic excitability) and how do neurons change their strength in communication (synaptic transmission) in an epileptic brain compared to a normal brain?”
The Chung lab seeks to have a better understanding of the pathogenesis of epilepsy. Understanding the pathogenesis is critical to developing novel therapeutic interventions and early diagnostics for epilepsy. “How does a neuron change itself to produce excessive electrical signals (intrinsic excitability) and how do neurons change their strength in communication (synaptic transmission) in an epileptic brain compared to a normal brain?”
The clue comes from the fact that inherited and de novo epilepsy is associated with mutations in ion channels, which are pore-forming proteins that generate electric current by mediating the flow of ions across the plasma membrane. They regulate electrical signals in morphologically and functionally distinct neuronal compartments: axons and dendrites. Axons deliver electrical signals to other neurons by action potentials whereas dendrites receive them at intercellular junctions called synapses. Ion channels enriched in axons are important for action potential initiation and termination, whereas ion channels enriched at synapses such as glutamate receptors are important for communication between neurons.
Since ion channels are critical regulators of neuronal activity, the two major long-term goals of Chung lab research have been to:
- Understand how epilepsy mutations affect ion channel function and lead to neuronal hyperactivity in inherited or de novo epilepsy.
- Identify molecular mechanisms that persistently alter ion channel function to cause hyperactivity during the development of acquired epilepsy.
To achieve these goals, Chung lab employs interdisciplinary approaches including primary neuronal culture, microscopy, biochemistry, electrophysiology and transgenic mice.
Our Principal Investigator is Hee Jung Chung.
Click the tabs below to learn more about our research and check us out at our website here!
Pathogenic mechanisms underlying epilepsy mutations in Kv7 channels
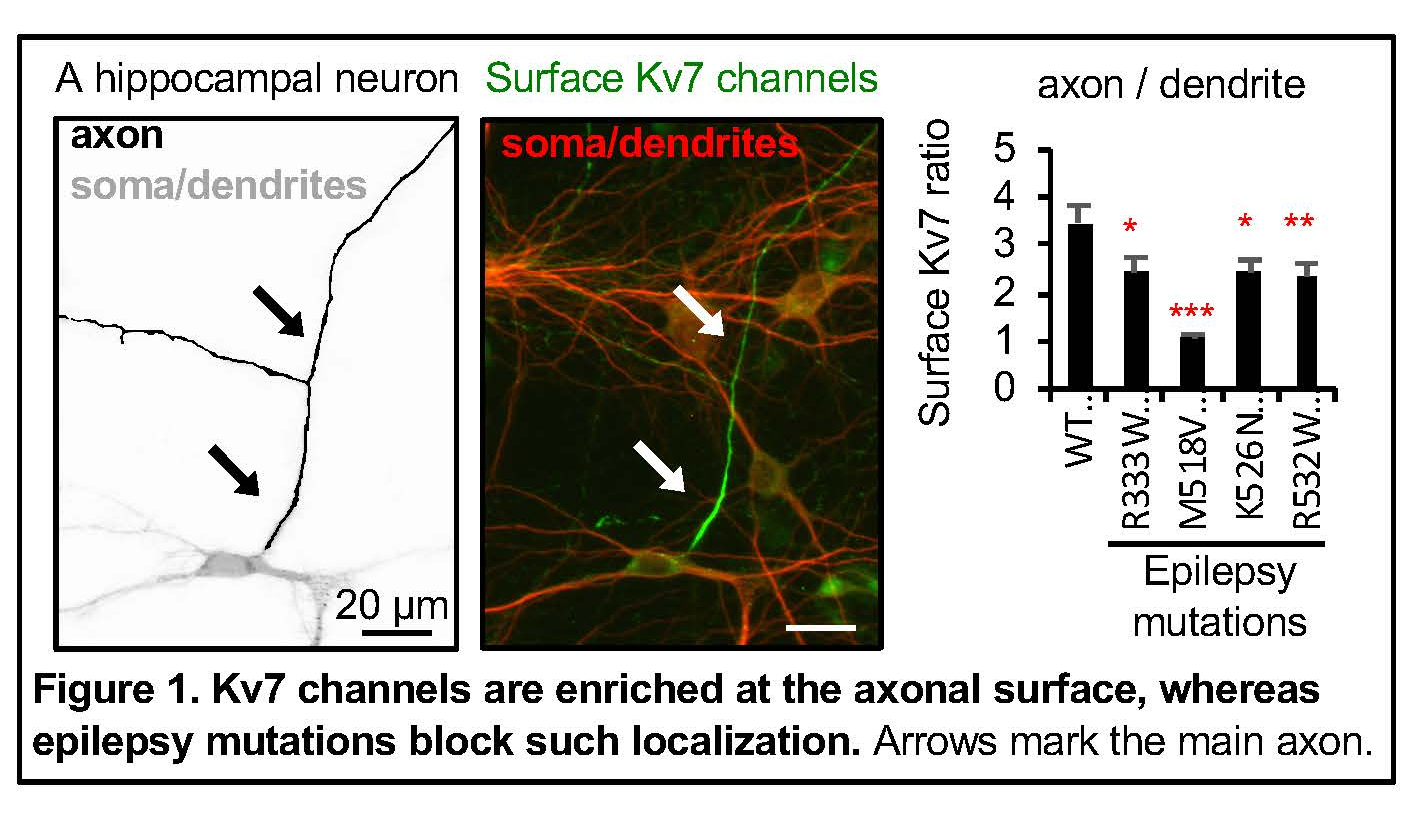 Neuronal Kv7/KCNQ potassium channels are heterotetramers composed of Kv7.2/KCNQ2 and Kv7.3/KCNQ3 subunits (Brown and Passmore, 2009), which are found throughout the brain including the hippocampus and neocortex (Cooper et al., 2001; Pan et al., 2006). Highly concentrated at the axonal initial segments (Pan et al., 2006) and preferentially enriched at the axonal plasma membrane compared to somatodendritic surface (Chung et al., 2006), these channels give rise to slowly activating and non-inactivating voltage-dependent outward potassium currents which potently inhibit both the repetitive and the burst firing of action potentials (Brown and Passmore, 2009).
Neuronal Kv7/KCNQ potassium channels are heterotetramers composed of Kv7.2/KCNQ2 and Kv7.3/KCNQ3 subunits (Brown and Passmore, 2009), which are found throughout the brain including the hippocampus and neocortex (Cooper et al., 2001; Pan et al., 2006). Highly concentrated at the axonal initial segments (Pan et al., 2006) and preferentially enriched at the axonal plasma membrane compared to somatodendritic surface (Chung et al., 2006), these channels give rise to slowly activating and non-inactivating voltage-dependent outward potassium currents which potently inhibit both the repetitive and the burst firing of action potentials (Brown and Passmore, 2009).
The physiological significance of these channels is underscored by the fact that dominant mutations in their subunits cause early-onset epilepsy including benign familial neonatal epilepsy (BFNE) and epileptic encephalopathy (EE) (Maljevic et al., 2010; Weckhuysen et al., 2012; Millichap et al., 2016). In contrast to transient appearance of seizures in neonates with BFNE (Psenka and Holden, 1996), EE is characterized by drug-resistant seizures, psychomotor retardation, developmental delay, and neuroradiological abnormalities including white matter reduction and enlarged ventricles (Saitsu et al., 2012; Weckhuysen et al., 2012; Millichap et al., 2016). Since the mapping of the first BFNE variants to Kcnq2 gene in 1998 (Biervert et al., 1998; Charlier et al., 1998; Singh et al., 1998), > 200 mutations in Kcnq2 and Kcnq3 genes have been associated with epilepsy.
However, how these epilepsy mutations of Kcnq2 and Kcnq3 ultimately lead to epilepsy with variable clinical severity remain largely elusive. How does a neuron change itself to produce excessive electrical signals in an epileptic brain compared to a normal brain?
One major research goal in Chung lab is therefore to determine the pathologic mechanisms underlying epilepsy mutations of Kcnq2 and Kcnq3. Specifically, we explore the following:
- Do epilepsy mutations affect voltage-dependent activation and axonal targeting of Kv7 channels and disrupt their ability to inhibit neuronal excitability?
- How does the disruption of Kv7 channel function and expression ultimately lead to epilepsy?
- Some EE mutations also cause autism spectrum disorder (ASD). The Chung lab has recently discovered that heterozygous loss of Kv7.2 leads to decreased sociability and increased repetitive behaviors in mice, which are reminiscent of ASD. Furthermore, heterozygous knock-out of Kv7.2 also causes enhanced exploratory activity and social dominant behaviors (Kim et al, 2019, in press). The Chung Lab is currently exploring the behavioral consequences of these mice as well as EE mutation knock-in mice to understand how disruption of these channels leads to intellectual disability and autism in addition to recurrent seizures.
Identification of molecular mechanisms underlying homeostatic intrinsic plasticity
Electrical properties of neuronal membranes can undergo persistent modification in response to neuronal activity or sensory experience. Homeostatic plasticity is one such mechanism by which neurons adapt their electrical activity within a physiologic range based on their previous activity. For example, a 2-day blockade of neuronal activity in the hippocampus leads to a compensatory increase in neuronal communication at synapses (termed synaptic transmission) as well as the ability of hippocampal neurons to fire action potentials at a given input signal (termed intrinsic excitability). Interestingly, activity blockade in the hippocampus for 2-4 weeks leads to temporal lobe epilepsy in rodents.
These findings raise a compelling question: “When and how do neurons exploit homeostatic plasticity to stabilize their network as a normal adaptive response, or to cause persistent neuronal hyperexcitability as a pathological manifestation in epilepsy?”
To answer this question, we must first understand how homeostatic plasticity is induced in the normal brain. Whereas much has been learned about homeostatic regulation of synaptic transmission (homeostatic synaptic plasticity), our knowledge of homeostatic regulation of intrinsic excitability (homeostatic intrinsic plasticity) remains largely unknown.
Chung lab has identified the signaling pathways underlying homeostatic control of intrinsic excitability in cultured hippocampal neurons, which are distinct from homeostatic synaptic scaling (Lee and Chung, 2014; Lee et al., 2015). Using unbiased gene expression profiling, we have identified 873 genes whose protein products could potentially mediate homeostatic plasticity which includes multiple potassium channel genes (Lee et al., 2015).
Chung lab is currently exploring the upstream signaling pathways that regulate multiple potassium channel genes as well as downstream effects of their transcript levels on neuronal excitability. In addition, Chung lab is actively developing novel transgenic mouse lines in which activity blockade can be induced in hippocampal neurons without involving invasive surgical methodologies. These mice will be useful in investigating the potential link between homeostatic plasticity and epileptogenesis.
Identification of novel players in neuronal hyperexcitability diseases including epilepsy
STEP61 weakens excitatory synapses by dephosphorylating and inactivating its substrates including synaptic glutamate receptors and kinases implicated in regulating synaptic transmission. However, its role in homeostatic plasticity and epilepsy remains unknown. We discovered that STEP61 mediates homeostatic plasticity of excitatory synaptic strength by modulating tyrosine phosphorylation of AMPA and NMDA receptors (Jang et al., 2015; Jang et al., 2016). We reported that a single acute episode of electroconvulsive seizures (ECS) increases expression of STEP61, amyloid-β (Aβ), and its precursor protein and decreases tyrosine phosphorylation of NMDA receptors (Jang et al., 2016), suggesting that elevated expression of STEP61 and Aβ may contribute to compensatory weakening of synaptic strength in response to seizures.
Given that the development of drug-resistant seizures in TLE is associated with sclerosis at or close to the seizure foci, we are currently studying the role of STEP61 in hippocampal hyperexcitability and sclerosis of TLE and investigating novel substrates of STEP61 that are involved in this process. These studies may provide insights into the pathogenesis of TLE and may provide STEP61 as a therapeutic target for TLE.